Research
The Yamago Lab pursues “brilliant molecules” in organic synthesis and polymer chemistry focusing on radical species, living radical polymerization, heteroatom-embedded compounds, organometallic compounds, curved aromatic molecules, and functional materials. Our goal is synthesis of attractive macromolecules, previously dream for chemists, in highly-controlled manner. These structurally well-defined macromolecules often show superior properties, and may be in near future they have importance similar to nucleic acid or proteins. We are also investigating routes using synthetic polymers based on bottom-up strategy to develop functional nanomaterials.
We have currently two major research interests listed below. The motivation of them is fundamental research based on our original molecules or reactions. Our aim is to yield practical application of the material used our compounds as a key.
Control of radical reaction and radical polymerization
Radical is an important active species similar to anion and cation, however, there are only a few methods to control their reactivity. The use of organoheteroatom compounds as precursors for carbon radical species give us a break-through to develop novel radical reactions and highly controlled radical polymerization. For example, the organoheteroatom compounds possessing tellurium, antimony, or bismuth atom in the following figure are successfully exploited for the living radical polymerization to control the molecular weight and the molecular weight distribution of polymers. Furthermore, we have revealed that these reactive organoheteroatom compounds are able to control structure of each polymer chain by precise block copolymerization and/or end-functionalization. Not only the reaction developments, but we have found that our polymerization method has great potential to synthesize functionalized polymer materials through collaborative work. Please see for detail in recent review (http://pubs.acs.org/doi/pdf/10.1021/cr9001269). We, therefore, feel proud to develop such novel and valuable compound with versatile activity and applications.

In living radical polymerization, the control is achieved by keeping “livingness” of reactivity during polymerization process by reversible generation of polymer end radical from precursor dormant species. Therefore, proper choice of heteroatom is quite important. In our polymerization methods, the equilibrium comes from degenerative chain transfer reaction. One of the key is quite high reactivity of organo-tellurium, -stibine, and -bismuthine compounds toward this transfer reaction. And recently we have found that the diheteroatom compounds such as ditelluride, distibine, and thiobismuthine, where heteroatoms are bound with σ-bond, work as effective co-controlling agent of living radical polymerization. This is a new methodology to control radical reactions. Now, we are investigating detailed reaction mechanism of this new reactions.
Synthesis of cyclic π-conjugated molecules

Figure 1. Structures of cycloparaphenylene and carbon nanotube
Cycloparaphenylenes (CPPs) are hoop-shaped π-conjugated molecules in which paraphenylene units are linked in a cyclic manner. CPPs are the shortest structural unit of armchair carbon nanotubes (CNTs) and are also structural constituents of fullerenes. Because of their unique structure, CPPs have attracted the attention of theoretical, physical, and synthetic chemists for more than half a century (Fig. 1). Despite the potential impact of CPPs in fundamental and applied chemistry and materials science, a lack of synthetic methods for their preparation have inhibited progress in unveiling their unique properties until recently.
Our group developed a new synthetic route toward CPPs and reported the selective synthesis of [8]CPP in 2010. The synthesis relies on the formation of “platinum square”, which was prepared by the transmetalation of 4,4′-bisstannylated biphenyl and Pt(cod)Cl2. Reductive elimination of the platinum species from platinum complex afforded [8]CPP (Fig. 2). Application of this synthetic method permitted selective synthesis of various CPPs such as [4]cyclo-2,7-pyrenylene and a cage-like three dimensional π-conjugated molecule (Fig. 3). This assembly/reductive elimination strategy would provide a variety of new cyclic π-conjugated molecules with different structures and topologies, which are challenging to obtain by conventional synthetic methods. Please see for detail in recent review [pdf].
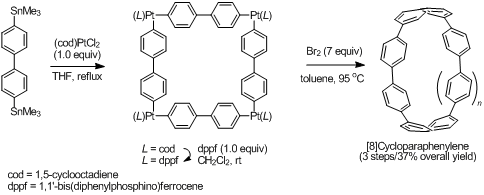
Figure 2. Synthesis of [8]cycloparaphenylene
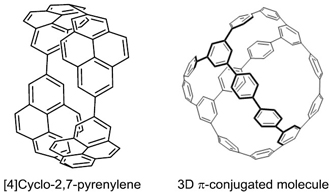
Figure 3. Various CPP derivatives
